MedicalDevice-v3.3.1(2020EN)
Inhoud
- 1 General information
- 2 Metadata
- 3 Revision History
- 4 Concept
- 5 Purpose
- 6 Evidence Base
- 7 Information Model
- 8 Example Instances
- 9 References
- 10 Valuesets
- 11 This information model in other releases
- 12 Information model references
- 13 Technical specifications in HL7v3 CDA and HL7 FHIR
- 14 Downloads
- 15 About this information
General information
Name: nl.zorg.MedicalDevice ![]()
Version: 3.3.1
HCIM Status:Final
Release: 2020
Release status: Published
Release date: 01-09-2020
Attention!! For this HCIM an erratum exists. You can find it in the Errata section of the release.
Metadata
| DCM::CoderList | Kerngroep Registratie aan de Bron |
| DCM::ContactInformation.Address | * |
| DCM::ContactInformation.Name | * |
| DCM::ContactInformation.Telecom | * |
| DCM::ContentAuthorList | Projectgroep Generieke Overdrachtsgegevens & Kerngroep Registratie aan de Bron |
| DCM::CreationDate | 2-1-2013 |
| DCM::DeprecatedDate | |
| DCM::DescriptionLanguage | nl |
| DCM::EndorsingAuthority.Address | |
| DCM::EndorsingAuthority.Name | PM |
| DCM::EndorsingAuthority.Telecom | |
| DCM::Id | 2.16.840.1.113883.2.4.3.11.60.40.3.10.1 |
| DCM::KeywordList | medisch hulpmiddel, implantaat |
| DCM::LifecycleStatus | Final |
| DCM::ModelerList | Kerngroep Registratie aan de Bron |
| DCM::Name | nl.zorg.MedischHulpmiddel |
| DCM::PublicationDate | 01-09-2020 |
| DCM::PublicationStatus | Published |
| DCM::ReviewerList | Projectgroep Generieke Overdrachtsgegevens & Kerngroep Registratie aan de Bron |
| DCM::RevisionDate | 19/05/2020 |
| DCM::Supersedes | nl.zorg.MedischHulpmiddel-v3.3 |
| DCM::Version | 3.3.1 |
| HCIM::PublicationLanguage | EN |
Revision History
Only available in Dutch
Publicatieversie 1.0 (15-02-2013) -
Publicatieversie 1.1 (01-07-2013)
| ZIB-11 | Gebruik van de GS1 standaard |
Publicatieversie 1.2 (01-04-2015)
| ZIB-83 | Wijzigingsvoorstel OverdrachtMedischHulpmiddel |
| ZIB-88 | OverdrachtMedischHulpmiddel |
| ZIB-110 | Example of the instrument van OverdrachtMedischHulpmiddel bevat kolom Zorgverlener, definities binnen deze kolom moeten duidelijker |
| ZIB-249 | In de klinische bouwsteen OverdrachtMedischHulpmiddel DCM::ValueSet SNOMED CT aangepast van concept AnatomischeLocalisatie naar tagged value DCM::ContentExpression. |
| ZIB-250 | In klinische bouwsteen OverdrachtMedischHulpmiddel de waarde van DCM::Name aanpassen van OverdrachtMedischHulpmiddel naar nl.nfu.OverdrachtMedischHulpmiddel. |
| ZIB-251 | Tagged value DCM::ValueSet van concept ProductType aangepast naar DCM::CodeSystem. |
| ZIB-252 | Tagged value DCM::ValueSet GTIN aangepast naar DCM::AssigningAuthority GTIN. |
| ZIB-308 | Prefix Overdracht weggehaald bij de generieke bouwstenen |
| ZIB-327 | AnatomischeLocalisatie wijzigen in AnatomischeLocatie |
| ZIB-353 | Tagged values DCM::CodeSystem aanpassen naar DCM::ValueSet incl. gekoppelde codelijst. |
Incl. algemene wijzigingsverzoeken:
| ZIB-94 | Aanpassen tekst van Disclaimer, Terms of Use & Copyrights |
| ZIB-154 | Consequenties opsplitsing Medicatie bouwstenen voor overige bouwstenen. |
| ZIB-200 | Naamgeving SNOMED CT in tagged values klinische bouwstenen gelijk getrokken. |
| ZIB-201 | Naamgeving OID: in tagged value notes van klinische bouwstenen gelijk getrokken. |
| ZIB-309 | EOI aangepast |
| ZIB-324 | Codelijsten Name en Description beginnen met een Hoofdletter |
| ZIB-326 | Tekstuele aanpassingen conform de kwaliteitsreview kerngroep 2015 |
Publicatieversie 3.0 (01-05-2016)
| ZIB-453 | Wijziging naamgeving ZIB's en logo's door andere opzet van beheer |
Publicatieversie 3.1 (04-09-2017)
| ZIB-457 | Uitbreiding AnatomischeLocatieCodelijst met Snomed qualifiers |
| ZIB-461 | Uitbreiding ProductIDCodelijst met HIBC |
| ZIB-517 | Onduidelijk hoe ProductTypeCodelijst gebruikt moet worden. |
| ZIB-522 | Codestelsel veranderen voor producttype |
| ZIB-547 | Toevoegen SNOMED CT als mogelijk codestelsel voor het coderen van producttype |
| ZIB-549 | De Engelse naam van de bouwsteen en het rootconcept zijn niet correct |
| ZIB-564 | Aanpassing/harmonisatie Engelse conceptnamen |
| ZIB-568 | Example Instances niet correct |
| ZIB-573 | Toevoegen van de mogelijkheid om dimensies van het hulpmiddel aan te geven |
| ZIB-574 | Alleen verwijzen naar het rootconcept van de ZIB. |
| ZIB-578 | Aan het concept anatomische locatie moet een concept Laterariteit toegevoegd worden. |
| ZIB-585 | Toevoegen concept Lateraliteit aan het concept Anatomische Locatie |
Publicatieversie 3.1.1 (01-10-2018)
| ZIB-673 | Tekstueel wijzigen 'medical aid' naar 'medical device'. |
Publicatieversie 3.2 (26-02-2019)
| ZIB-680 | Toevoegen van einddatum bij medisch hulpmiddel |
Publicatieversie 3.3 (06-07-2019)
| ZIB-734 | Geen informatie |
Publicatieversie 3.3.1 (01-09-2020)
| ZIB-1116 | Geen informatie |
| ZIB-1135 | referentie naar codesysteem UNSPSC in issue verwijderen. |
| ZIB-1120 | Typo medisch hulpmiddel |
Concept
Medical devices are any internally implanted and external devices and/or aids used by the patient (in the past) to reduce the effects of functional limitations in organ systems or to facilitate the treatment of a disease.
Purpose
Data on medical devices is recorded for several reasons. Knowledge of the presence of these implants enables tracing and taking the aid or device into account in diagnostic or therapeutic procedures, care and transport.
Examples include:
- Consequences for transportation, toilet use, etc., in the case of a wheelchair;
- A pacemaker can be of medical importance, but also has consequences for planning radiological exams.
Evidence Base
Recording data on medically complex devices such as pacemakers is not yet common in EPD systems in the Netherlands, but is sometimes lacking: a letter from a specialist for example often does not include information on which type of pacemaker the patient has (and from which manufacturer).
The Dutch Ministry of Health, Welfare and Sport (VWS) will pass legislation for a national basic register of implants. Every healthcare center will have to supply a UDI (Unique Device Identification, with a link to GTIN) and a UPI (Unique Patient Identification) to the basic register. This will prevent situations in which a large group of patients have an aid or implant in which problems have been detected that cannot be traced.
Information Model
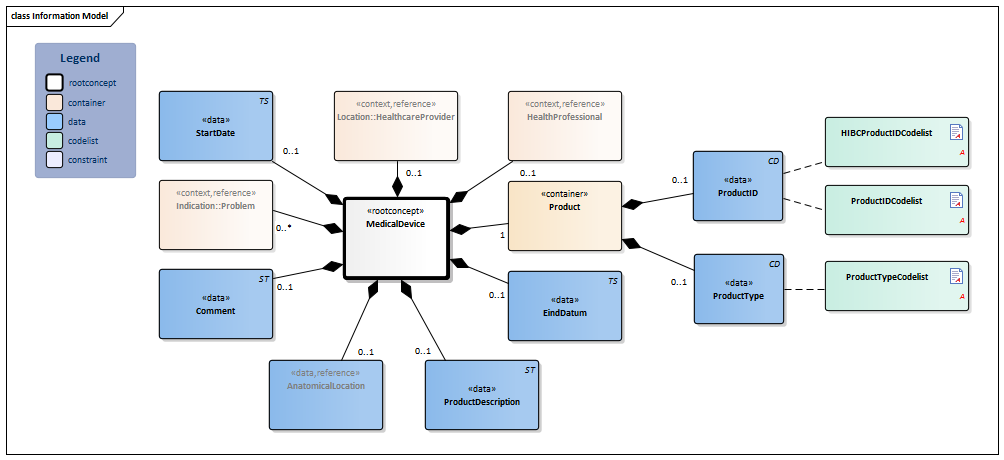
| Type | Id | Concept | Card. | Definition | DefinitionCode | Reference | |||||||||
| NL-CM:10.1.1 | Root concept of the MedicalDevice information model. This root concept contains all data elements of the MedicalDevice information model. |
|
|||||||||||||
| NL-CM:10.1.2 | 1 | The medical device (internally or externally). |
|
||||||||||||
| NL-CM:10.1.4 | 0..1 | Unique identification of the product, such as the serial number.
Frequently used coding systems are HIBC and GTIN. If the law requires this to be registered on the basis of a UDI (Unique Device Identifier), the unique identification must consist of a UDI-DI (Device Identifier) and a UDI-PI (Production Identifier(s)). See http://www.gs1.org/healthcare/udi for more information. The UDI-DI must be recorded in reference to GS1 GTIN (01) encryptions, with which for example a firm is linked to the product type. The UDI-PI must consist of the following: application identifier (AI); expiration date (17) and serial number (21) and/or batch or lot number (10). |
| ||||||||||||
| NL-CM:10.1.3 | 0..1 | The code of the type of product. |
| ||||||||||||
| NL-CM:10.1.13 | 0..1 | Textual description of the product. | |||||||||||||
| NL-CM:10.1.15 | 0..1 | Patient’s anatomical location of the medical device used. |
|
| |||||||||||
| NL-CM:10.1.7 | 0..* | The medical reason for use of the medical device. |
| ||||||||||||
| NL-CM:10.1.11 | 0..1 | The start date of the first use or implant of the medical device. A ‘vague’ date, such as only the year, is permitted. | |||||||||||||
| NL-CM:10.1.14 | 0..1 | The end date of the last use or explant of the medical device. A ‘vague’ date, such as only the year, is permitted. | |||||||||||||
| NL-CM:10.1.10 | 0..1 | Comment about use or information on the medical device used. |
|
||||||||||||
| NL-CM:10.1.8 | 0..1 | The healthcare provider at which use of the medical device was initiated or where the aid was implanted. |
| ||||||||||||
| NL-CM:10.1.9 | 0..1 | The healthcare provider involved in the indication for use of the medical device implant. |
| ||||||||||||
Columns Concept and DefinitionCode: hover over the values for more information
For explanation of the symbols, please see the legend page ![]()
Example Instances
Only available in Dutch
| Begin Datum |
Product | Anatomische Locatie | Lateraliteit | Indicatie | Locatie | Toelichting | ||
| ProductID | ProductType | ProbleemNaam | Organisatie Naam |
Afdeling Specialisme |
||||
| 08-03-2012 | GTIN/HIBC code | Rolstoel | Multiple sclerose | Kan korte afstanden lopen | ||||
| Begin Datum |
Product | Anatomische Locatie | Lateraliteit | Lateraliteit | Locatie | Toelichting | ||
| ProductID | ProductType | ProbleemNaam | Organisatie Naam |
Afdeling Specialisme |
||||
| 2007 | GTIN/HIBC code | Gehoorapparaat | Oor | Rechts | Presbyacusis | St. Franciscus Gasthuis | Audiologie | Apparaat niet zichtbaar (diep in de gehooringang) |
| Begin Datum |
Product | Anatomische Locatie | Lateraliteit | Indicatie | Locatie | Toelichting | ||
| ProductID | ProductType | ProbleemNaam | Organisatie Naam |
Afdeling Specialisme |
||||
| 10-02-2004 | GTIN/HIBC code | Pacemaker | Subclavian pouch | Links | Paroxymaal boezemfibrilleren | Academisch Medisch Centrum | Cardiologie | Laatst doorgemeten in 2011 |
References
1. Kamerbrief over het voorstel voor een register van implantaten. [Online] Beschikbaar op: http://www.rijksoverheid.nl/documenten-en-publicaties/kamerstukken/2012/11/20/kamerbrief-over-het-voorstel-voor-een-register-van-implantaten.html [Geraadpleegd: 15 september 2014].
Valuesets
HIBCProductIDCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.10.1.5 | Binding: Required |
| Conceptname | Codesystem name | Codesystem OID |
| Alle waarden | Health Industry Bar Code (HIBC) | 2.16.840.1.113883.6.40 |
ProductIDCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.10.1.3 | Binding: Required |
| Conceptname | Codesystem name | Codesystem OID |
| Alle waarden | Global Trade Item Number (GTIN) | 1.3.160 |
ProductTypeCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.10.1.1 | Binding: Required |
| Conceptname | Codesystem name | Codesystem OID |
| SNOMED CT: <260787004|Physical object| | SNOMED CT | 2.16.840.1.113883.6.96 |
This information model in other releases
- Release 2015, (Version 1.2)
- Release 2016, (Version 3.0)
- Release 2017, (Version 3.1)
- Prerelease 2018-2, (Version 3.2)
- Prerelease 2019-2, (Version 3.3)
- Prerelease 2021-2, (Version 3.4)
- Prerelease 2022-1, (Version 3.5)
- Prerelease 2023-1, (Version 4.0)
- Prerelease 2024-1, (Versie 4.0)
- Release 2024, (Version 5.0)
Information model references
This information model refers to
This information model is used in
- AbilityToPerformMouthcareActivities-v3.1
- BladderFunction-v3.2
- BowelFunction-v3.1.1
- FeedingTubeSystem-v3.3
- FunctionalOrMentalStatus-v3.2
- HearingFunction-v3.2
- Infusion-v3.3
- Mobility-v3.3
- NursingIntervention-v3.2
- Procedure-v5.2
- Respiration-v3.2
- Stoma-v3.3
- VisualFunction-v3.1
- Wound-v3.3
Technical specifications in HL7v3 CDA and HL7 FHIR
To exchange information based on health and care information models, additional, more technical specifications are required.
Not every environment can handle the same technical specifications. For this reason, there are several types of technical specifications:
- HL7® version 3 CDA compatible specifications, available through the Nictiz ART-DECOR® environment

- HL7® FHIR® compatible specifications, available through the Nictiz environment on the Simplifier FHIR

Downloads
This information model is also available as pdf file ![]() or as spreadsheet
or as spreadsheet ![]()
About this information
The information in this wikipage is based on Release 2020
SNOMED CT and LOINC codes are based on:
- SNOMED Clinical Terms version: 20200731 [R] (July 2020 Release)
- LOINC version 2.67
Conditions for use are located on the mainpage ![]()
This page is generated on 29/09/2020 21:34:53 with ZibExtraction v. 4.0.7577.31095