MedicationDispense-v3.0(2016EN)
Inhoud
General information
Name: nl.zorg.MedicationDispense ![]()
Version: 3.0
HCIM Status:Final
Release: 2016
Release status: Published
Release date: 1-5-2016
Metadata
| DCM::CoderList | Kerngroep Registratie aan de Bron |
| DCM::ContactInformation.Address | |
| DCM::ContactInformation.Name | * |
| DCM::ContactInformation.Telecom | |
| DCM::ContentAuthorList | Projectgroep Generieke Overdrachtsgegevens & Kerngroep Registratie aan de Bron |
| DCM::CreationDate | 19-12-2013 |
| DCM::DeprecatedDate | |
| DCM::DescriptionLanguage | nl |
| DCM::EndorsingAuthority.Address | |
| DCM::EndorsingAuthority.Name | PM |
| DCM::EndorsingAuthority.Telecom | |
| DCM::Id | 2.16.840.1.113883.2.4.3.11.60.40.3.9.4 |
| DCM::KeywordList | Medicatie, Verstrekking |
| DCM::LifecycleStatus | Final |
| DCM::ModelerList | Kerngroep Registratie aan de Bron |
| DCM::Name | nl.zorg.MedicatieVerstrekking |
| DCM::PublicationDate | 1-5-2016 |
| DCM::PublicationStatus | Published |
| DCM::ReviewerList | Projectgroep Generieke Overdrachtsgegevens & Kerngroep Registratie aan de Bron |
| DCM::RevisionDate | 1-4-2015 |
| DCM::Superseeds | nl.nfu.MedicatieVerstrekking-v1.0 |
| DCM::Version | 3.0 |
| HCIM::PublicationLanguage | EN |
Revision History
Only available in Dutch
Publicatieversie 1.0 (01-04-2015)
| ZIB-56 | RFC Bouwsteen Medicatie |
| ZIB-308 | Prefix Overdracht weggehaald bij de generieke bouwstenen |
Incl. algemene wijzigingsverzoeken:
| ZIB-94 | Aanpassen tekst van Disclaimer, Terms of Use & Copyrights |
| ZIB-154 | Consequenties opsplitsing Medicatie bouwstenen voor overige bouwstenen. |
| ZIB-200 | Naamgeving SNOMED CT in tagged values klinische bouwstenen gelijk getrokken. |
| ZIB-201 | Naamgeving OID: in tagged value notes van klinische bouwstenen gelijk getrokken. |
| ZIB-309 | EOI aangepast |
| ZIB-324 | Codelijsten Name en Description beginnen met een Hoofdletter |
| ZIB-326 | Tekstuele aanpassingen conform de kwaliteitsreview kerngroep 2015 |
Publicatieversie 3.0 (01-05-2016)
.
Concept
Medication supply describes the supply of a medicinal product to a specific patient (or the administerer or a representative), with the intention of it being used according to the included instructions (usually to honor the supply request in a medication prescription).
Medication supply takes place when the patient (or any of the others listed above) physically receives the product.
Purpose
Recording supply information can be an important step in drawing up an up-to-date medication profile.
Information Model
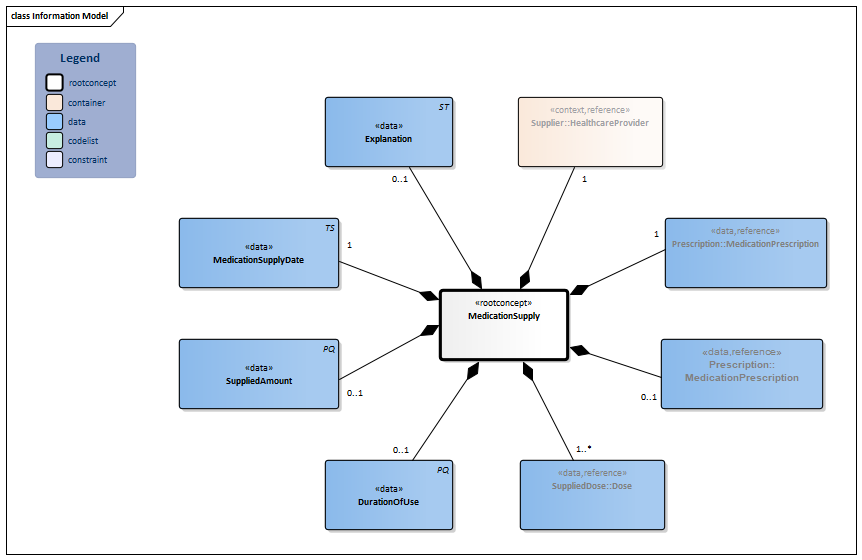
| Type | Id | Concept | Card. | Definition | DefinitionCode | Reference | |||||||
| NL-CM:9.4.1 | Root concept of the MedicationSupply information model. This root concept contains all data elements of the MedicationSupply information model. | ||||||||||||
| NL-CM:9.4.3 | 1 | The supplied product is usually a medicine. Strictly speaking, food, blood products, aids and bandages do not fall under the category of medication, but supplying these products can be reported in the same manner.
In most cases, the supplied product will match the product prescribed in the medication prescription. In principle, this will be the prescribed product, but the supplier may substitute it by replacing the prescribed product with an equivalent product. Furthermore, the supplier can replace a generically formulated prescribed product by a trade product, or they can replace the prescribed trade product with a different trade product. |
| ||||||||||
| NL-CM:9.4.4 | 0..1 | An agreement or order for the use of medication. |
| ||||||||||
| NL-CM:9.4.5 | 1..* | Instructions for the administerer to administer the medication (the patient themselves, a nurse or other aid).
The supplied dose will constitute more specified details of the administering schedule from the prescription, especially in terms of the administering schedule. For example, the prescription could contain 4xd1, which is then translated by the supplier into a label text as ‘1 unit to be taken 4 times a day, during meals’. In the case of Baxter packs, these kinds of codes are translated from ‘4xd1’ to specific times, such as 9:00am, 12:00pm, etc. |
| ||||||||||
| NL-CM:9.4.6 | 0..1 | The period in which the medication is expected to be used. The value depends on the dose and the amount supplied. | |||||||||||
| NL-CM:9.4.8 | 0..1 | Number of units of the product (measured based on the relevant product code) supplied. | |||||||||||
| NL-CM:9.4.7 | 1 | The date and time at which the medication was supplied. | |||||||||||
| NL-CM:9.4.2 | 1 | In almost all cases, the supplier will be a registered pharmacist. In principle, it could also be supplied by a webshop (in case of an online order), a drugstore or a foreign pharmacy. |
| ||||||||||
| NL-CM:9.4.9 | 0..1 | Comments on supplying the medication. | |||||||||||
Columns Concept and DefinitionCode: hover over the values for more information
For explanation of the symbols, please see the legend page ![]()
Example Instances
Only available in Dutch
| VerstrekkingsProduct | DatumMedicatie Verstrekking | Verstrekte Hoeveelheid | VerbruiksDuur | VerstrekkingsDosering | |
| ProductNaam | Keerdosis | Toedieningsschema | ToedieningsWeg | |||
| Paracetamol tablet 500 mg | 3-5-2012 | 100 tabletten | 4 weken | Zo nodig 500mg (=1st), max. 4x/dag | oraal |
| VerstrekkingsProduct | DatumMedicatie Verstrekking | Verstrekte Hoeveelheid | VerbruiksDuur | VerstrekkingsDosering | |
| ProductNaam | Keerdosis | Toedieningsschema | ToedieningsWeg | |||
| Pantoprazol injpdr 40mg fl | 11-9-2012 0:00 | 5 flacons | 5 dagen | 1x/dag(8u) 40mg (=1st) | iv |
| VerstrekkingsProduct | DatumMedicatie Verstrekking | Verstrekte Hoeveelheid | VerbruiksDuur | VerstrekkingsDosering | |
| ProductNaam | Keerdosis | Toedieningsschema | ToedieningsWeg | |||
| Dalteparine 2500 injvlst 12.500 ie/ml wwsp 0,2ml | 19-9-2012 | 90 stuks | 90 dagen | 1x/dag(18u) 2500ie(=0,2ml) | subcutaan |
| VerstrekkingsProduct | DatumMedicatie Verstrekking | Verstrekte Hoeveelheid | VerbruiksDuur | VerstrekkingsDosering | |
| ProductNaam | AanvullendeToelichting | ToedieningsWeg | |||
| Prednison 5mg | 1-9-2012 | 100 stuks | Volgens afbouwschema | oraal |
References
1. GROOT, E. (2011) Dataset medicatieproces 2011. [Online] Den Haag: Nictiz. Beschikbaar op: http://www.nictiz.nl/module/360/590/Dataset_Medicatieproces_2011.xlsx [Geraadpleegd: 23 juli 2014].
2. HL7v3-implementatiehandleiding medicatieproces versie 6.1.0.0. [Online] Den Haag: Nictiz. Beschikbaar op: http://www.nictiz.nl/uploaded/FILES/html_cabinet/live/Zorgtoepassing/Medicatieproces/AORTA_Mp_IH_Medicatieproces_HL7.htm [Geraadpleegd: 23 juli 2014].
3. Dossier Medicatieoverzicht. [Online] Beschikbaar op: Oria.nl. [Geraadpleegd: 23 juli 2014].
4. G-standaard documentatie. [Online] Beschikbaar op: http://www.z-index.nl/ [Geraadpleegd: 23 juli 2014].
This information model in other releases
More on this information model
To exchange information based on health and care information models, additional, more technical specifications are required.
Not every environment can handle the same technical specifications. For this reason, there are several types of technical specifications:
- HL7® version 3 CDA compatible specifications, available through the Nictiz ART-DECOR® environment

- HL7® FHIR® compatible specifications, available through the Nictiz environment on the Simplifier FHIR

This information model is also available as pdf file ![]() or as spreadsheet
or as spreadsheet ![]()
About this information
The information in this wikipage is based on Release summer 2016
Conditions for use are located on the mainpage ![]()
This page is generated on 24/01/2018 23:30:24