AdministrationAgreement-v1.0.1(2017EN)
Inhoud
General information
Name: nl.zorg.AdministrationAgreement ![]()
Version: 1.0.1
HCIM Status:Final
Release: 2017
Release status: Published
Release date: 31-12-2017
Metadata
| DCM::CoderList | Projectgroep Medicatieproces |
| DCM::ContactInformation.Address | |
| DCM::ContactInformation.Name | * |
| DCM::ContactInformation.Telecom | |
| DCM::ContentAuthorList | Projectgroep Medicatieproces |
| DCM::CreationDate | 1-2-2017 |
| DCM::DeprecatedDate | |
| DCM::DescriptionLanguage | nl |
| DCM::EndorsingAuthority.Address | |
| DCM::EndorsingAuthority.Name | PM |
| DCM::EndorsingAuthority.Telecom | |
| DCM::Id | 2.16.840.1.113883.2.4.3.11.60.40.3.9.8 |
| DCM::KeywordList | Medicatie, Toediening |
| DCM::LifecycleStatus | Final |
| DCM::ModelerList | Architectuurgroep Registratie aan de Bron |
| DCM::Name | nl.zorg.Toedieningsafspraak |
| DCM::PublicationDate | 31-12-2017 |
| DCM::PublicationStatus | Published |
| DCM::ReviewerList | Projectgroep Medicatieproces & Architectuurgroep Registratie aan de Bron |
| DCM::RevisionDate | 31-12-2017 |
| DCM::Superseeds | nl.zorg.MedicatieVerstrekking-v3.0 |
| DCM::Version | 1.0.1 |
| HCIM::PublicationLanguage | EN |
Revision History
Only available in Dutch
Publicatieversie 1.0 (04-09-2017)
Publicatieversie 1.0.1 (31-12-2017)
| ZIB-643 | Kleine tekstuele verbeteringen |
Concept
An administration agreement is the use (or administering) instructions from the pharmacist to the patient (or their representative or administrator), whereby a medication agreement is structured at a concrete level.
Purpose
The goal of the administration agreement is to provide insight into the concrete instructions for administration/use of medication.
Information Model
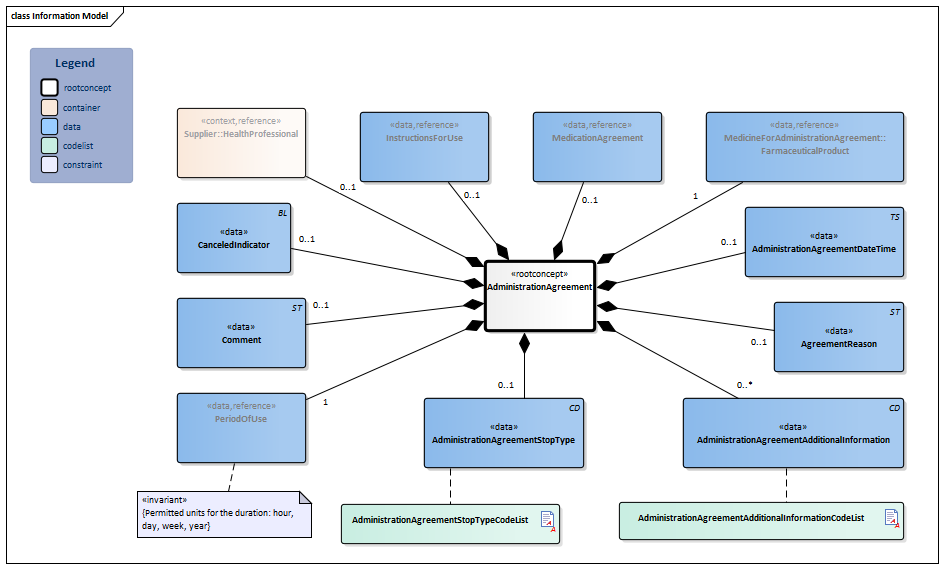
| Type | Id | Concept | Card. | Definition | DefinitionCode | Reference | |||||||
| NL-CM:9.8.20132 | Root concept of the AdministrationAgreement information model. This root concept contains all data elements of the AdministrationAgreement information model. | ||||||||||||
| NL-CM:9.8.22097 | 0..1 | The supplier (pharmacist) that entered the administration agreement. |
| ||||||||||
| NL-CM:9.8.20237 | 1 | Medicine in the AdministrationAgreement. |
| ||||||||||
| NL-CM:9.8.22098 | 0..1 | Instructions for administering the medication, e.g. dose and route of administration. |
| ||||||||||
| NL-CM:9.8.20133 | 0..1 | The time at which the agreement was made. | |||||||||||
| NL-CM:9.8.22499 | 0..1 | Reason for this agreement. This will often be the same reason as the one for the medication agreement.
This field has the option to - if applicable - enter a specific reason for the administration agreement. Examples include: substitution, transfer to GDS, patient request for different product, etc. |
|||||||||||
| NL-CM:9.8.22660 | 1 | Start date: This is the time at which the agreement was to take effect (or took effect or will take effect). This is the time at which the instructions for use in this agreement start. In the case of an agreement to discontinue use, this is the start date of the original administration agreement. The end date indicates from when the medication is to be discontinued.
Duration: The intended duration of use. E.g. 5 days or 8 weeks. It is not allowed to indicate the duration in months, because different months have a variable duration in days. End date: The time at which the period of use ends (or ended or will end). In the case of an agreement to discontinue use, this is the time at which the medication is to be discontinued. To avoid confusion between 'to' and 'up to', the submission of time is always mandatory for the end date. With medication for an indefinite period only a start date is indicated. |
| ||||||||||
| NL-CM:9.8.22394 | 0..1 | Relationship to the medication agreement on which the administration agreement is based. |
| ||||||||||
| NL-CM:9.8.22498 | 0..1 | Stop type, the manner in which this medication is discontinued (temporary or definitive). |
| ||||||||||
| NL-CM:9.8.23034 | 0..1 | In the event of an error correction, this indicator is to be put on for the incorrect agreement. | |||||||||||
| NL-CM:9.8.23284 | 0..* | Additional information includes details on the structure of the agreement made.
This element mainly contains information that until now has been structured with ZZ rules. The medication-related topics that are now supported by the ZZ rules are best supported with a system functionality. A process has been started for this by Z-index/KNMP. For now, the following list will be used. This list will be replaced by a thesaurus in the G standard at a later stage. |
| ||||||||||
| NL-CM:9.8.22275 | 0..1 | Comments on the administration agreement. |
|
||||||||||
Columns Concept and DefinitionCode: hover over the values for more information
For explanation of the symbols, please see the legend page ![]()
Example Instances
Only available in Dutch
| ToedieningsAfspraakDatumTijd | Gebruiksperiode | GeneesmiddelBijToedieningsafspraak | Verstrekker | GeannuleerdIndicator | ToedieningsafspraakStoptype | ||
| Ingangsdatum | Einddatum | Duur | ProductCode | Zorgaanbieder | |||
| 2-5-2012 12:14:09 | 02-05-12 | 4 weken | Paracetamol tablet 500 mg | Apotheek De Gulle Gaper | |||
| 10-9-2015 9:16:31 | 5 dagen | Pantoprazol injpdr 40 mg fl | Apotheek De Gulle Gaper | Ja | |||
| 19-9-2014 7:12:59 | 19-09-14 | 18-12-14 | Dalteparine 2500 injvlst 12.500 ie/ml wwsp 0,2ml | Poli-apotheek Het Ziekenhuis | |||
| 30-8-2013 8:41:43 | 01-08-13 | 30-08-13 | Prednison 5 mg | Poli-apotheek Het Ziekenhuis | Definitief |
| Gebruiksinstructie | ||||||
| Omschrijving | ToedieningsWeg | HerhaalperiodeCyclischSchema | Doseerinstructie | |||
| Doseerduur | Dosering|Keerdosis | Toedieningsschema|Frequentie|Interval|Toedientijd|Weekdag|Dagdeel | Zo nodig | |||
| Vanaf 2 mei gedurende 4 weken zo nodig bij pijn 500 mg (=1 st), max. 4x per dag | oraal | 1 | 4 weken | 1 stuk | max. 4x/dag | Bij pijn |
| Gedurende 5 dagen 1x/dag (8u) 40 mg (=1 st) | iv | 40 mg (=1 st) | 1x per dag om 8.00 uur | |||
| Vanaf 19-9-2014 tot 18-12-2014 1x per dag om 18uur 2500ie(=0,2ml) | subcutaan | 2500 IE (=0,2 ml) | 1x per dag om 18.00 uur | |||
| Van 1-8-2013 tot 30-8-2013 volgens afbouwschema. Per 30-8-2013 onderbreken. | oraal | 1 |
Valuesets
AdministrationAgreementAdditionalInformationCodeList
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.9.8.2 | Binding: |
| Conceptname | Conceptcode | Codesystem name | Codesystem OID | Description |
| Geneesmiddel niet verstrekt | 1 | Voorlopige aanvullende informatie toedieningsafspraak | 2.16.840.1.113883.2.4.3.11.60.20.77.5.2.3.999 | Geneesmiddel niet verstrekt |
| Niet leverbaar | 2 | Voorlopige aanvullende informatie toedieningsafspraak | 2.16.840.1.113883.2.4.3.11.60.20.77.5.2.3.999 | Niet leverbaar |
| Medicatieafspraak gewijzigd: substantie | 3 | Voorlopige aanvullende informatie toedieningsafspraak | 2.16.840.1.113883.2.4.3.11.60.20.77.5.2.3.999 | Medicatieafspraak gewijzigd: substantie |
| Medicatieafspraak gewijzigd: dosering | 4 | Voorlopige aanvullende informatie toedieningsafspraak | 2.16.840.1.113883.2.4.3.11.60.20.77.5.2.3.999 | Medicatieafspraak gewijzigd: dosering |
| Medicatieafspraak gewijzigd: farmaceutische vorm | 5 | Voorlopige aanvullende informatie toedieningsafspraak | 2.16.840.1.113883.2.4.3.11.60.20.77.5.2.3.999 | Medicatieafspraak gewijzigd: farmaceutische vorm |
| Medicatieafspraak gewijzigd: verwisseling van persoon | 6 | Voorlopige aanvullende informatie toedieningsafspraak | 2.16.840.1.113883.2.4.3.11.60.20.77.5.2.3.999 | Medicatieafspraak gewijzigd: verwisseling van persoon |
| Machtiging aangevraagd | 7 | Voorlopige aanvullende informatie toedieningsafspraak | 2.16.840.1.113883.2.4.3.11.60.20.77.5.2.3.999 | Machtiging aangevraagd |
| Machtiging afgewezen | 8 | Voorlopige aanvullende informatie toedieningsafspraak | 2.16.840.1.113883.2.4.3.11.60.20.77.5.2.3.999 | Machtiging afgewezen |
| Machtiging in gebruik nemen | 9 | Voorlopige aanvullende informatie toedieningsafspraak | 2.16.840.1.113883.2.4.3.11.60.20.77.5.2.3.999 | Machtiging in gebruik nemen |
| Gebruiksinstructie gegeven | 10 | Voorlopige aanvullende informatie toedieningsafspraak | 2.16.840.1.113883.2.4.3.11.60.20.77.5.2.3.999 | Gebruiksinstructie gegeven |
| Controle inhalatietechniek | 11 | Voorlopige aanvullende informatie toedieningsafspraak | 2.16.840.1.113883.2.4.3.11.60.20.77.5.2.3.999 | Controle inhalatietechniek |
| Meter periodieke controle | 12 | Voorlopige aanvullende informatie toedieningsafspraak | 2.16.840.1.113883.2.4.3.11.60.20.77.5.2.3.999 | Meter periodieke controle |
| Pen controle/omruil | 13 | Voorlopige aanvullende informatie toedieningsafspraak | 2.16.840.1.113883.2.4.3.11.60.20.77.5.2.3.999 | Pen controle/omruil |
| Bijwerking besproken | 14 | Voorlopige aanvullende informatie toedieningsafspraak | 2.16.840.1.113883.2.4.3.11.60.20.77.5.2.3.999 | Bijwerking besproken |
| Afhandeling bijwerking niet-lareb | 15 | Voorlopige aanvullende informatie toedieningsafspraak | 2.16.840.1.113883.2.4.3.11.60.20.77.5.2.3.999 | Afhandeling bijwerking niet-lareb |
| Melding lareb | 16 | Voorlopige aanvullende informatie toedieningsafspraak | 2.16.840.1.113883.2.4.3.11.60.20.77.5.2.3.999 | Melding lareb |
| Medicatiebewaking OT | 17 | Voorlopige aanvullende informatie toedieningsafspraak | 2.16.840.1.113883.2.4.3.11.60.20.77.5.2.3.999 | Medicatiebewaking OT |
AdministrationAgreementStopTypeCodeList
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.9.8.1 | Binding: |
| Conceptname | Conceptcode | Codesystem name | Codesystem OID | Description |
| Tijdelijk | 1 | StopType | 2.16.840.1.113883.2.4.3.11.60.20.77.5.2.1 | Tijdelijke onderbreking van medicamenteuze behandeling (bijvoorbeeld tijdelijk stoppen gebruik vanwege operatie). |
| Definitief | 2 | StopType | 2.16.840.1.113883.2.4.3.11.60.20.77.5.2.1 | Het staken van een bestaande medicamenteuze behandeling. |
This information model in other releases
- Prerelease 2018-2, (Version 1.0.1)
- Prerelease 2019-2, (Version 1.0.2)
- Release 2020, (Version 1.0.3)
- Prerelease 2021-2, (Version 1.1)
- Prerelease 2022-1, (Version 2.0)
- Prerelease 2023-1, (Version 2.1)
- Prerelease 2024-1, (Version 3.0)
Information model references
This information model refers to
- HealthcareProvider-v3.1.1
- InstructionsForUse-v1.1
- MedicationAgreement-v1.0.1
- PharmaceuticalProduct-v2.0
- TimeInterval-v1.0
This information model is used in
Technical specifications in HL7v3 CDA and HL7 FHIR
To exchange information based on health and care information models, additional, more technical specifications are required.
Not every environment can handle the same technical specifications. For this reason, there are several types of technical specifications:
- HL7® version 3 CDA compatible specifications, available through the Nictiz ART-DECOR® environment

- HL7® FHIR® compatible specifications, available through the Nictiz environment on the Simplifier FHIR

Downloads
This information model is also available as pdf file ![]() or as spreadsheet
or as spreadsheet ![]()
About this information
The information in this wikipage is based on Release 2017
Conditions for use are located on the mainpage ![]()
This page is generated on 21/12/2018 14:11:39 with ZibExtraction v. 3.0.6929.24609