MedicationUse2-v1.0(2017EN): verschil tussen versies
(Nieuwe pagina aangemaakt met '==General information== Name: '''nl.zorg.MedicationUse2''' link=MedicatieGebruik2-v1.0(2017NL)<BR> Version: '''1.0''' <br> HCIM Status:Final<b...') |
(Copy of) |
||
| (4 tussenliggende versies door dezelfde gebruiker niet weergegeven) | |||
| Regel 3: | Regel 3: | ||
Version: '''1.0''' <br> | Version: '''1.0''' <br> | ||
HCIM Status:Final<br> | HCIM Status:Final<br> | ||
| − | + | Release: '''2017''' <br> | |
Release status: Prepublished<br> | Release status: Prepublished<br> | ||
| − | + | Release date: 04-09-2017 | |
----- | ----- | ||
<div style="text-align: right; direction: ltr; margin-left: 1em;" >[[Bestand: Back 16.png| link= HCIM_Release_2017(EN)]] [[HCIM_Release_2017(EN) |Back to HCIM list ]]</div> | <div style="text-align: right; direction: ltr; margin-left: 1em;" >[[Bestand: Back 16.png| link= HCIM_Release_2017(EN)]] [[HCIM_Release_2017(EN) |Back to HCIM list ]]</div> | ||
| Regel 74: | Regel 74: | ||
==Information Model== | ==Information Model== | ||
<BR> | <BR> | ||
| − | + | <imagemap> Bestand:MedicationUse2-v1.0Model(EN).png | center | |
| + | rect 179 91 291 155 [[HealthProfessional-v3.1(2017EN)]] | ||
| + | rect 496 480 731 540 [[#ReasonForChangeOrDiscontinuationOfUseCodList]] | ||
| + | rect 762 480 962 540 [[#MedicationUseStopTypeCodeList]] | ||
| + | rect 745 373 887 443 [[#13447]] | ||
| + | rect 629 91 741 161 [[#13449]] | ||
| + | rect 479 91 591 155 [[InstructionsForUse-v1.0(2017EN)]] | ||
| + | rect 775 236 886 300 [[#13450]] | ||
| + | rect 329 91 441 155 [[Product-v1.0(2017EN)]] | ||
| + | rect 774 93 886 163 [[#13455]] | ||
| + | rect 170 229 312 299 [[#13448]] | ||
| + | rect 513 374 684 444 [[#13445]] | ||
| + | rect 333 374 475 444 [[TimeInterval-v1.0(2017EN)]] | ||
| + | rect 166 373 308 443 [[#13451]] | ||
| + | rect 488 229 599 299 [[#13453]] | ||
| + | desc none | ||
| + | </imagemap> | ||
<BR> | <BR> | ||
{| border= "1" width="1500px" style = "font-size: 9.5pt; border: solid 1px silver; border-collapse:collapse;" cellpadding = "3px" cellspacing ="0px" | {| border= "1" width="1500px" style = "font-size: 9.5pt; border: solid 1px silver; border-collapse:collapse;" cellpadding = "3px" cellspacing ="0px" | ||
| Regel 80: | Regel 96: | ||
|style="width:30px;"|Type||style="width:100px;"|Id||colspan="6 " style="width:140px;"|Concept||Card.||style="width: 600px;"|Definition||style="width:200px;"|DefinitionCode||style="width:200px;"|Reference | |style="width:30px;"|Type||style="width:100px;"|Id||colspan="6 " style="width:140px;"|Concept||Card.||style="width: 600px;"|Definition||style="width:200px;"|DefinitionCode||style="width:200px;"|Reference | ||
|-style="vertical-align:top; background-color: #E3E3E3; " | |-style="vertical-align:top; background-color: #E3E3E3; " | ||
| − | |style = "text-align:center" |[[Bestand: block.png| 20px]] | + | |style = "text-align:center" |[[Bestand: block.png| 20px | link=]] |
||NL-CM:9.11.21338 | ||NL-CM:9.11.21338 | ||
| − | |colspan ="6" style ="padding-left: 0px"|<span Title="NL: MedicatieGebruik">[[Bestand: arrowdown.png | 10px]]MedicationUse</span> | + | |colspan ="6" style ="padding-left: 0px"|<span Id=13453 Title="NL: MedicatieGebruik">[[Bestand: arrowdown.png | 10px | link=]]MedicationUse</span> |
| | | | ||
|Root concept of the MedicationUse information model. This root concept contains all data elements of the MedicationUse information model. | |Root concept of the MedicationUse information model. This root concept contains all data elements of the MedicationUse information model. | ||
| Regel 88: | Regel 104: | ||
| | | | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
| − | |style = "text-align:center" |[[Bestand: Verwijzing.png| 20px]] | + | |style = "text-align:center" |[[Bestand: Verwijzing.png| 20px | link=]] |
||NL-CM:9.11.23290 | ||NL-CM:9.11.23290 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
| − | |colspan ="5" style ="padding-left: 0px"|<span Title="NL: Voorschrijver::Zorgverlener">[[Bestand: arrowright.png | 10px]]Prescriber::HealthProfessional</span> | + | |colspan ="5" style ="padding-left: 0px"|<span Id=13452 Title="NL: Voorschrijver::Zorgverlener">[[Bestand: arrowright.png | 10px | link=]]Prescriber::HealthProfessional</span> |
|0..1 | |0..1 | ||
|The health professional that entered the medication agreement with the patient. | |The health professional that entered the medication agreement with the patient. | ||
| Regel 98: | Regel 114: | ||
{| | {| | ||
|- | |- | ||
| − | |[[Bestand: block.png]]||[[HealthProfessional-v3.1(2017EN) |HealthProfessional]] | + | |[[Bestand: block.png | link=HealthProfessional-v3.1(2017EN)]]||[[HealthProfessional-v3.1(2017EN) |HealthProfessional]] |
|} | |} | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
| − | |style = "text-align:center" |[[Bestand: Verwijzing.png| 20px]] | + | |style = "text-align:center" |[[Bestand: Verwijzing.png| 20px | link=]] |
||NL-CM:9.11.21339 | ||NL-CM:9.11.21339 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
| − | |colspan ="5" style ="padding-left: 0px"|<span Title="NL: Gebruiksproduct::Product">[[Bestand: arrowright.png | 10px]]ProductUsed::Product</span> | + | |colspan ="5" style ="padding-left: 0px"|<span Id=13454 Title="NL: Gebruiksproduct::Product">[[Bestand: arrowright.png | 10px | link=]]ProductUsed::Product</span> |
|1 | |1 | ||
|The product used. This is usually medication. Food, blood products, aids and bandages do not strictly fall under the category of medication, but can be recorded as well. | |The product used. This is usually medication. Food, blood products, aids and bandages do not strictly fall under the category of medication, but can be recorded as well. | ||
| Regel 113: | Regel 129: | ||
{| | {| | ||
|- | |- | ||
| − | |[[Bestand: block.png]]||[[Product-v1.0(2017EN) |Product]] | + | |[[Bestand: block.png | link=Product-v1.0(2017EN)]]||[[Product-v1.0(2017EN) |Product]] |
|} | |} | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
| − | |style = "text-align:center" |[[Bestand: Verwijzing.png| 20px]] | + | |style = "text-align:center" |[[Bestand: Verwijzing.png| 20px | link=]] |
||NL-CM:9.11.22504 | ||NL-CM:9.11.22504 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
| − | |colspan ="5" style ="padding-left: 0px"|<span Title="NL: Gebruiksinstructie">[[Bestand: arrowright.png | 10px]]InstructionsForUse</span> | + | |colspan ="5" style ="padding-left: 0px"|<span Id=13457 Title="NL: Gebruiksinstructie">[[Bestand: arrowright.png | 10px | link=]]InstructionsForUse</span> |
|0..1 | |0..1 | ||
|Instructions for the use of the medication, e.g. dose and route of administration. In the event of medication use, this is the pattern of use established by the patient or which the patient followed. | |Instructions for the use of the medication, e.g. dose and route of administration. In the event of medication use, this is the pattern of use established by the patient or which the patient followed. | ||
| Regel 126: | Regel 142: | ||
{| | {| | ||
|- | |- | ||
| − | |[[Bestand: block.png]]||[[InstructionsForUse-v1.0(2017EN) |InstructionsForUse]] | + | |[[Bestand: block.png | link=InstructionsForUse-v1.0(2017EN)]]||[[InstructionsForUse-v1.0(2017EN) |InstructionsForUse]] |
|} | |} | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
| − | |style = "text-align:center" |[[Bestand: TS.png| 16px]] | + | |style = "text-align:center" |[[Bestand: TS.png| 16px | link=]] |
||NL-CM:9.11.22398 | ||NL-CM:9.11.22398 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
| − | |colspan ="5" style ="padding-left: 0px"|<span Title="NL: MedicatieGebruikDatumTijd">[[Bestand: arrowright.png | 10px]]MedicationUseDateTime</span> | + | |colspan ="5" style ="padding-left: 0px"|<span Id=13451 Title="NL: MedicatieGebruikDatumTijd">[[Bestand: arrowright.png | 10px | link=]]MedicationUseDateTime</span> |
|1 | |1 | ||
|Date on which this use is entered. | |Date on which this use is entered. | ||
| Regel 138: | Regel 154: | ||
| | | | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
| − | |style = "text-align:center" |[[Bestand: Verwijzing.png| 20px]] | + | |style = "text-align:center" |[[Bestand: Verwijzing.png| 20px | link=]] |
||NL-CM:9.11.22663 | ||NL-CM:9.11.22663 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
| − | |colspan ="5" style ="padding-left: 0px"|<span Title="NL: Gebruiksperiode::TijdsInterval">[[Bestand: arrowright.png | 10px]]PeriodOfUse</span> | + | |colspan ="5" style ="padding-left: 0px"|<span Id=13456 Title="NL: Gebruiksperiode::TijdsInterval">[[Bestand: arrowright.png | 10px | link=]]PeriodOfUse</span> |
|0..1 | |0..1 | ||
|<b>Start date</b>: This is the time at which the agreement was to take effect (or took effect or will take effect). | |<b>Start date</b>: This is the time at which the agreement was to take effect (or took effect or will take effect). | ||
| Regel 150: | Regel 166: | ||
{| | {| | ||
|- | |- | ||
| − | |[[Bestand: block.png]]||[[ | + | |[[Bestand: block.png | link=TimeInterval-v1.0(2017EN)]]||[[TimeInterval-v1.0(2017EN) |TimeInterval]] |
|} | |} | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
| − | |style = "text-align:center" |[[Bestand: BL.png| 16px]] | + | |style = "text-align:center" |[[Bestand: BL.png| 16px | link=]] |
||NL-CM:9.11.22492 | ||NL-CM:9.11.22492 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
| − | |colspan ="5" style ="padding-left: 0px"|<span Title="NL: VolgensAfspraakIndicator">[[Bestand: arrowright.png | 10px]]AsAgreedIndicator</span> | + | |colspan ="5" style ="padding-left: 0px"|<span Id=13448 Title="NL: VolgensAfspraakIndicator">[[Bestand: arrowright.png | 10px | link=]]AsAgreedIndicator</span> |
|0..1 | |0..1 | ||
|Is the medicine used as outlined in the medication agreement? | |Is the medicine used as outlined in the medication agreement? | ||
| Regel 162: | Regel 178: | ||
| | | | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
| − | |style = "text-align:center" |[[Bestand: BL.png| 16px]] | + | |style = "text-align:center" |[[Bestand: BL.png| 16px | link=]] |
||NL-CM:9.11.22399 | ||NL-CM:9.11.22399 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
| − | |colspan ="5" style ="padding-left: 0px"|<span Title="NL: GebruikIndicator">[[Bestand: arrowright.png | 10px]]UseIndicator</span> | + | |colspan ="5" style ="padding-left: 0px"|<span Id=13455 Title="NL: GebruikIndicator">[[Bestand: arrowright.png | 10px | link=]]UseIndicator</span> |
|1 | |1 | ||
|Is this medicine used or not? | |Is this medicine used or not? | ||
| Regel 171: | Regel 187: | ||
| | | | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
| − | |style = "text-align:center" |[[Bestand: ST.png| 16px]] | + | |style = "text-align:center" |[[Bestand: ST.png| 16px | link=]] |
||NL-CM:9.11.22491 | ||NL-CM:9.11.22491 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
| − | |colspan ="5" style ="padding-left: 0px"|<span Title="NL: RedenGebruik">[[Bestand: arrowright.png | 10px]]ReasonForUse</span> | + | |colspan ="5" style ="padding-left: 0px"|<span Id=13449 Title="NL: RedenGebruik">[[Bestand: arrowright.png | 10px | link=]]ReasonForUse</span> |
|0..1 | |0..1 | ||
|The reason for using the medication, particularly in self-care medicine purchased by the patient themselves. | |The reason for using the medication, particularly in self-care medicine purchased by the patient themselves. | ||
| Regel 180: | Regel 196: | ||
| | | | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
| − | |style = "text-align:center" |[[Bestand: CD.png| 16px]] | + | |style = "text-align:center" |[[Bestand: CD.png| 16px | link=]] |
||NL-CM:9.11.23132 | ||NL-CM:9.11.23132 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
| − | |colspan ="5" style ="padding-left: 0px"|<span Title="NL: MedicatieGebruikStopType">[[Bestand: arrowright.png | 10px]]MedicationUseStopType</span> | + | |colspan ="5" style ="padding-left: 0px"|<span Id=13447 Title="NL: MedicatieGebruikStopType">[[Bestand: arrowright.png | 10px | link=]]MedicationUseStopType</span> |
|0..1 | |0..1 | ||
|Stop type, the manner in which this medication is discontinued (temporary or definitive). | |Stop type, the manner in which this medication is discontinued (temporary or definitive). | ||
| Regel 190: | Regel 206: | ||
{| | {| | ||
|- | |- | ||
| − | |[[Bestand: List2.png]]||[[#MedicationUseStopTypeCodeList|MedicationUseStopTypeCodeList]] | + | |[[Bestand: List2.png | link=#MedicationUseStopTypeCodeList]]||[[#MedicationUseStopTypeCodeList|MedicationUseStopTypeCodeList]] |
|} | |} | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
| − | |style = "text-align:center" |[[Bestand: CD.png| 16px]] | + | |style = "text-align:center" |[[Bestand: CD.png| 16px | link=]] |
||NL-CM:9.11.22493 | ||NL-CM:9.11.22493 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
| − | |colspan ="5" style ="padding-left: 0px"|<span Title="NL: RedenWijzigenOfStoppenGebruik">[[Bestand: arrowright.png | 10px]]ReasonForChangeOrDiscontinuationOfUse</span> | + | |colspan ="5" style ="padding-left: 0px"|<span Id=13445 Title="NL: RedenWijzigenOfStoppenGebruik">[[Bestand: arrowright.png | 10px | link=]]ReasonForChangeOrDiscontinuationOfUse</span> |
|0..* | |0..* | ||
|Reason for changing or discontinuing use of medication. | |Reason for changing or discontinuing use of medication. | ||
| Regel 203: | Regel 219: | ||
{| | {| | ||
|- | |- | ||
| − | |[[Bestand: List2.png]]||[[#ReasonForChangeOrDiscontinuationOfUseCodList|ReasonForChangeOrDiscontinuationOfUseCodList]] | + | |[[Bestand: List2.png | link=#ReasonForChangeOrDiscontinuationOfUseCodList]]||[[#ReasonForChangeOrDiscontinuationOfUseCodList|ReasonForChangeOrDiscontinuationOfUseCodList]] |
|} | |} | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
| − | |style = "text-align:center" |[[Bestand: ST.png| 16px]] | + | |style = "text-align:center" |[[Bestand: ST.png| 16px | link=]] |
||NL-CM:9.11.21624 | ||NL-CM:9.11.21624 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
| − | |colspan ="5" style ="padding-left: 0px"|<span Title="NL: Toelichting">[[Bestand: arrowright.png | 10px]]Comment</span> | + | |colspan ="5" style ="padding-left: 0px"|<span Id=13450 Title="NL: Toelichting">[[Bestand: arrowright.png | 10px | link=]]Comment</span> |
|0..1 | |0..1 | ||
|Comments on the medication use. | |Comments on the medication use. | ||
| Regel 227: | Regel 243: | ||
{|class="wikitable" width="1316px" style= "font-size: 9.5pt;" | {|class="wikitable" width="1316px" style= "font-size: 9.5pt;" | ||
|-style=vertical-align:top; | |-style=vertical-align:top; | ||
| − | | style="background-color: #1F497D; width: 18%; color | + | | style="background-color: #1F497D; width: 18%; "|<font color=#FFFFFF><b>MedicatieGebruik</b></font><font color=#FFFFFF><b> </b></font><font color=#FFFFFF><b>DatumTijd</b></font> |
| − | | style="background-color: #1F497D; width: 4%; color | + | | style="background-color: #1F497D; width: 4%; "|<font color=#FFFFFF><b>GebruikIndicator</b></font> |
| − | | style="background-color: #1F497D; width: 11%; color | + | | style="background-color: #1F497D; width: 11%; "|<font color=#FFFFFF><b>VolgensAfspraak</b></font><font color=#FFFFFF><b> </b></font><font color=#FFFFFF><b>Indicator</b></font> |
| − | | style="background-color: #1F497D; width: 11%; color | + | | style="background-color: #1F497D; width: 11%; "|<font color=#FFFFFF><b>Medicatiegebruik</b></font><font color=#FFFFFF><b> </b></font><font color=#FFFFFF><b>Stoptype</b></font> |
| − | |colspan="3" style="background-color: #1F497D; width: 27%; color | + | |colspan="3" style="background-color: #1F497D; width: 27%; "|<font color=#FFFFFF><b>Gebruiksperiode</b></font> |
| − | | style="background-color: #1F497D; width: 28%; color | + | | style="background-color: #1F497D; width: 28%; "|<font color=#FFFFFF><b>Gebruiksproduct</b></font> |
|-style=vertical-align:top; | |-style=vertical-align:top; | ||
| style="background-color: #548DD4; width: 18%; "| | | style="background-color: #548DD4; width: 18%; "| | ||
| Regel 238: | Regel 254: | ||
| style="background-color: #548DD4; width: 11%; "| | | style="background-color: #548DD4; width: 11%; "| | ||
| style="background-color: #548DD4; width: 11%; "| | | style="background-color: #548DD4; width: 11%; "| | ||
| − | | style="background-color: #548DD4; width: 9%; color | + | | style="background-color: #548DD4; width: 9%; "|<font color=#FFFFFF><b>Ingangsdatum</b></font> |
| − | | style="background-color: #548DD4; width: 8%; color | + | | style="background-color: #548DD4; width: 8%; "|<font color=#FFFFFF><b>Einddatum</b></font> |
| − | | style="background-color: #548DD4; width: 9%; color | + | | style="background-color: #548DD4; width: 9%; "|<font color=#FFFFFF><b>Gebruiksduur</b></font> |
| − | | style="background-color: #548DD4; width: 28%; color | + | | style="background-color: #548DD4; width: 28%; "|<font color=#FFFFFF><b>ProductCode</b></font> |
|-style=vertical-align:top; | |-style=vertical-align:top; | ||
| style="background-color: #D9D9D9; width: 18%; "| | | style="background-color: #D9D9D9; width: 18%; "| | ||
| Regel 252: | Regel 268: | ||
| style="background-color: #D9D9D9; width: 28%; "| | | style="background-color: #D9D9D9; width: 28%; "| | ||
|-style=vertical-align:top; | |-style=vertical-align:top; | ||
| − | | style="width: 18% | + | | style="width: 18%; "|3-6-2014 16:19:07 |
| style="width: 4%; "|Ja | | style="width: 4%; "|Ja | ||
| style="width: 11%; "| | | style="width: 11%; "| | ||
| Regel 259: | Regel 275: | ||
| style="width: 8%; "| | | style="width: 8%; "| | ||
| style="width: 9%; "|1 maand | | style="width: 9%; "|1 maand | ||
| − | | style="width: 28% | + | | style="width: 28%; "|Paracetamol tablet 500 mg |
|-style=vertical-align:top; | |-style=vertical-align:top; | ||
| − | | style="width: 18% | + | | style="width: 18%; "|11-9-2012 17:21:00 |
| style="width: 4%; "|Ja | | style="width: 4%; "|Ja | ||
| style="width: 11%; "|Ja | | style="width: 11%; "|Ja | ||
| style="width: 11%; "| | | style="width: 11%; "| | ||
| style="width: 9%; "|01-09-12 | | style="width: 9%; "|01-09-12 | ||
| − | | style="width: 8% | + | | style="width: 8%; "|05-09-12 |
| style="width: 9%; "| | | style="width: 9%; "| | ||
| − | | style="width: 28% | + | | style="width: 28%; "|Pantoprazol injpdr 40 mg fl |
|-style=vertical-align:top; | |-style=vertical-align:top; | ||
| − | | style="width: 18% | + | | style="width: 18%; "|19-9-2014 4:12:11 |
| style="width: 4%; "|Nee | | style="width: 4%; "|Nee | ||
| style="width: 11%; "|Nee | | style="width: 11%; "|Nee | ||
| style="width: 11%; "|Definitief | | style="width: 11%; "|Definitief | ||
| − | | style="width: 9% | + | | style="width: 9%; "|17-09-14 |
| style="width: 8%; "| | | style="width: 8%; "| | ||
| style="width: 9%; "| | | style="width: 9%; "| | ||
| − | | style="width: 28% | + | | style="width: 28%; "|Dalteparine 2500 injvlst 12.500 ie/ml wwsp 0,2ml |
|} | |} | ||
{|class="wikitable" width="1316px" style= "font-size: 9.5pt;" | {|class="wikitable" width="1316px" style= "font-size: 9.5pt;" | ||
|-style=vertical-align:top; | |-style=vertical-align:top; | ||
| − | | style="background-color: #1F497D; width: 11%; color | + | | style="background-color: #1F497D; width: 11%; "|<font color=#FFFFFF><b>RedenGebruik</b></font> |
| − | | style="background-color: #1F497D; width: 11%; color | + | | style="background-color: #1F497D; width: 11%; "|<font color=#FFFFFF><b>RedenWijzigen</b></font><font color=#FFFFFF><b> </b></font><font color=#FFFFFF><b>OfStoppen</b></font><font color=#FFFFFF><b> </b></font><font color=#FFFFFF><b>Gebruik</b></font> |
| − | |colspan="5" style="background-color: #1F497D; width: 59%; color | + | |colspan="5" style="background-color: #1F497D; width: 59%; "|<font color=#FFFFFF><b>GebruiksInstructie</b></font> |
| style="background-color: #1F497D; width: 19%; "| | | style="background-color: #1F497D; width: 19%; "| | ||
|-style=vertical-align:top; | |-style=vertical-align:top; | ||
| style="background-color: #548DD4; width: 11%; "| | | style="background-color: #548DD4; width: 11%; "| | ||
| style="background-color: #548DD4; width: 11%; "| | | style="background-color: #548DD4; width: 11%; "| | ||
| − | | style="background-color: #548DD4; width: 16%; color | + | | style="background-color: #548DD4; width: 16%; "|<font color=#FFFFFF><b>Omschrijving</b></font> |
| − | | style="background-color: #548DD4; width: 9%; color | + | | style="background-color: #548DD4; width: 9%; "|<font color=#FFFFFF><b>ToedieningsWeg</b></font> |
| − | | style="background-color: #548DD4; width: 11%; color | + | | style="background-color: #548DD4; width: 11%; "|<font color=#FFFFFF><b>Aanvullende instructie</b></font> |
| − | | style="background-color: #548DD4; width: 11%; color | + | | style="background-color: #548DD4; width: 11%; "|<font color=#FFFFFF><b>Doseerinstructie</b></font> |
| style="background-color: #548DD4; width: 12%; "| | | style="background-color: #548DD4; width: 12%; "| | ||
| style="background-color: #548DD4; width: 19%; "| | | style="background-color: #548DD4; width: 19%; "| | ||
| Regel 300: | Regel 316: | ||
| style="background-color: #D9D9D9; width: 9%; "| | | style="background-color: #D9D9D9; width: 9%; "| | ||
| style="background-color: #D9D9D9; width: 11%; "| | | style="background-color: #D9D9D9; width: 11%; "| | ||
| − | | style="background-color: #D9D9D9; width: 11% | + | | style="background-color: #D9D9D9; width: 11%; "|<b>Doseerduur</b> |
| − | | style="background-color: #D9D9D9; width: 12% | + | | style="background-color: #D9D9D9; width: 12%; "|<b>Dosering<noWiki>|</noWiki></b><b> </b><b>Keerdosis</b> |
| − | | style="background-color: #D9D9D9; width: 19% | + | | style="background-color: #D9D9D9; width: 19%; "|<b>Toedieningsschema</b><b> </b><b><noWiki>|</noWiki>Frequentie</b><b> </b><b><noWiki>|</noWiki>Interval</b><b> </b><b><noWiki>|</noWiki>Toedientijd</b><b> </b><b><noWiki>|</noWiki>Weekdag</b><b> </b><b><noWiki>|</noWiki>Dagdeel</b><BR> |
|-style=vertical-align:top; | |-style=vertical-align:top; | ||
| style="width: 11%; "|Pijn | | style="width: 11%; "|Pijn | ||
| style="width: 11%; "| | | style="width: 11%; "| | ||
| − | | style="width: 16% | + | | style="width: 16%; "|In de maand mei heb ik regelmatig paracetamol gebruikt. |
| style="width: 9%; "| | | style="width: 9%; "| | ||
| style="width: 11%; "| | | style="width: 11%; "| | ||
| Regel 315: | Regel 331: | ||
| style="width: 11%; "|Ulcusprofylaxe | | style="width: 11%; "|Ulcusprofylaxe | ||
| style="width: 11%; "| | | style="width: 11%; "| | ||
| − | | style="width: 16% | + | | style="width: 16%; "|Vanaf 1 september 2012 gedurende 5 dagen 1x per dag om 8uur 40 mg (=1 st) |
| style="width: 9%; "|iv | | style="width: 9%; "|iv | ||
| style="width: 11%; "| | | style="width: 11%; "| | ||
| style="width: 11%; "| | | style="width: 11%; "| | ||
| − | | style="width: 12% | + | | style="width: 12%; "|40 mg (=1 st) |
| style="width: 19%; "|1x per dag om 8.00 uur | | style="width: 19%; "|1x per dag om 8.00 uur | ||
|-style=vertical-align:top; | |-style=vertical-align:top; | ||
| style="width: 11%; "| | | style="width: 11%; "| | ||
| − | | style="width: 11% | + | | style="width: 11%; "|(Mogelijke) bijwerking |
| − | | style="width: 16% | + | | style="width: 16%; "|Tijdelijk gestopt vanwege toenemende bijwerkingen: duizeligheid en misselijkheid. |
| style="width: 9%; "|subcutaan | | style="width: 9%; "|subcutaan | ||
| style="width: 11%; "| | | style="width: 11%; "| | ||
| style="width: 11%; "| | | style="width: 11%; "| | ||
| − | | style="width: 12% | + | | style="width: 12%; "|2500 IE |
| − | | style="width: 19% | + | | style="width: 19%; "|1x per dag om 18.00 uur<BR> |
|} | |} | ||
| Regel 468: | Regel 484: | ||
| + | ==This information model in other releases== | ||
| + | --- | ||
==More on this information model== | ==More on this information model== | ||
| − | + | To exchange information based on health and care information models, additional, more technical specifications are required.<BR> | |
| − | This information model is also available as [[Media:nl.zorg.MedicationUse2-v1.0( | + | Not every environment can handle the same technical specifications. For this reason, there are several types of technical specifications: |
| + | <ul> | ||
| + | <li>HL7® version 3 CDA compatible specifications, available through the Nictiz ART-DECOR® environment [http://decor.nictiz.nl/art-decor/decor-scenarios--zib2017bbr-?id=2.16.840.1.113883.2.4.3.11.60.7.4.2.9.11&effectiveDate=2017-09-04T00:00:00&language=en-US&scenariotree=false [[File:artdecor.jpg|16px|link=]]]</li> | ||
| + | <li>HL7® FHIR® compatible specifications, available through the Nictiz environment on the Simplifier FHIR [https://simplifier.net/NictizSTU3/~resources?text=zib&category=Profile [[File:fhir.png|link=]]]</li> | ||
| + | </ul> | ||
| + | This information model is also available as [[Media:nl.zorg.MedicationUse2-v1.0(2017EN).pdf|pdf file]] [[File:PDF.png|link=]] or as [[Media:nl.zorg.MedicationUse2-v1.0(2017EN).xlsx|spreadsheet]] [[File:xlsx.png|link=]] | ||
==About this information== | ==About this information== | ||
The information in this wikipage is based on Prerelease 2017 #1 <BR> | The information in this wikipage is based on Prerelease 2017 #1 <BR> | ||
Conditions for use are located on the mainpage [[Bestand:list2.png|link=HCIM_Mainpage]]<BR> | Conditions for use are located on the mainpage [[Bestand:list2.png|link=HCIM_Mainpage]]<BR> | ||
| − | This page is generated on | + | This page is generated on 30/11/2017 13:37:32 <BR> |
----- | ----- | ||
<div style="text-align: right; direction: ltr; margin-left: 1em;" >[[Bestand: Back 16.png| link= HCIM_Release_2017(EN)]] [[HCIM_Release_2017(EN) |Back to HCIM list ]]</div> | <div style="text-align: right; direction: ltr; margin-left: 1em;" >[[Bestand: Back 16.png| link= HCIM_Release_2017(EN)]] [[HCIM_Release_2017(EN) |Back to HCIM list ]]</div> | ||
Huidige versie van 20 feb 2018 om 10:06
Inhoud
General information
Name: nl.zorg.MedicationUse2 ![]()
Version: 1.0
HCIM Status:Final
Release: 2017
Release status: Prepublished
Release date: 04-09-2017
Metadata
| DCM::CoderList | Projectgroep Medicatieproces |
| DCM::ContactInformation.Address | |
| DCM::ContactInformation.Name | * |
| DCM::ContactInformation.Telecom | |
| DCM::ContentAuthorList | Projectgroep Medicatieproces |
| DCM::CreationDate | 1-2-2017 |
| DCM::DeprecatedDate | |
| DCM::DescriptionLanguage | nl |
| DCM::EndorsingAuthority.Address | |
| DCM::EndorsingAuthority.Name | PM |
| DCM::EndorsingAuthority.Telecom | |
| DCM::Id | 2.16.840.1.113883.2.4.3.11.60.40.3.9.11 |
| DCM::KeywordList | Medicatie, Gebruik |
| DCM::LifecycleStatus | Final |
| DCM::ModelerList | Architectuurgroep Registratie aan de Bron |
| DCM::Name | nl.zorg.MedicatieGebruik2 |
| DCM::PublicationDate | 04-09-2017 |
| DCM::PublicationStatus | Prepublished |
| DCM::ReviewerList | Projectgroep Medicatieproces & Architectuurgroep Registratie aan de Bron |
| DCM::RevisionDate | 04-09-2017 |
| DCM::Superseeds | nl.zorg.MedicatieGebruik-v3.0 |
| DCM::Version | 1.0 |
| HCIM::PublicationLanguage | EN |
Revision History
Only available in Dutch
Publicatieversie 1.0 (04-09-2017).
Concept
MedicationUse is a statement on the historic, current or intended use of a certain medicine.
Purpose
The goal of the medication use is to provide insight into the patient’s pattern of use.
Information Model
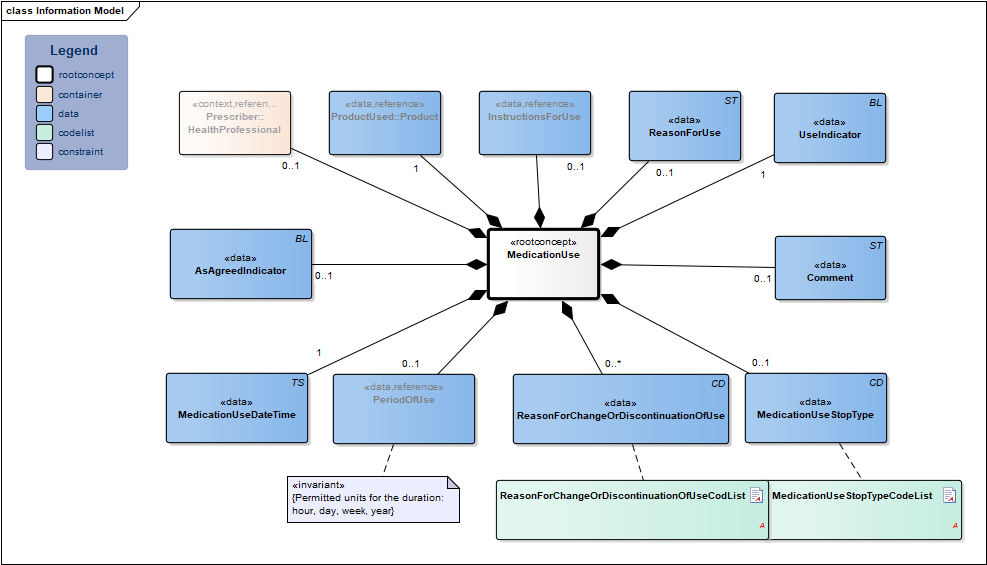
| Type | Id | Concept | Card. | Definition | DefinitionCode | Reference | |||||||
| NL-CM:9.11.21338 | Root concept of the MedicationUse information model. This root concept contains all data elements of the MedicationUse information model. | ||||||||||||
| NL-CM:9.11.23290 | 0..1 | The health professional that entered the medication agreement with the patient. |
| ||||||||||
| NL-CM:9.11.21339 | 1 | The product used. This is usually medication. Food, blood products, aids and bandages do not strictly fall under the category of medication, but can be recorded as well.
In principle, this will be the prescribed product, but the product used may differ from the prescribed product. |
| ||||||||||
| NL-CM:9.11.22504 | 0..1 | Instructions for the use of the medication, e.g. dose and route of administration. In the event of medication use, this is the pattern of use established by the patient or which the patient followed. |
| ||||||||||
| NL-CM:9.11.22398 | 1 | Date on which this use is entered. | |||||||||||
| NL-CM:9.11.22663 | 0..1 | Start date: This is the time at which the agreement was to take effect (or took effect or will take effect).
Duration: The intended duration of use. E.g. 5 days or 8 weeks. It is not allowed to indicate the duration in months, because different months have a variable duration in days. End date: The time at which the period of use ends (or ended or will end). To avoid confusion between 'to' and 'up to', the submission of time is always mandatory for the end date. |
| ||||||||||
| NL-CM:9.11.22492 | 0..1 | Is the medicine used as outlined in the medication agreement? | |||||||||||
| NL-CM:9.11.22399 | 1 | Is this medicine used or not? | |||||||||||
| NL-CM:9.11.22491 | 0..1 | The reason for using the medication, particularly in self-care medicine purchased by the patient themselves. | |||||||||||
| NL-CM:9.11.23132 | 0..1 | Stop type, the manner in which this medication is discontinued (temporary or definitive). |
| ||||||||||
| NL-CM:9.11.22493 | 0..* | Reason for changing or discontinuing use of medication. |
| ||||||||||
| NL-CM:9.11.21624 | 0..1 | Comments on the medication use. |
|
||||||||||
Columns Concept and DefinitionCode: hover over the values for more information
For explanation of the symbols, please see the legend page ![]()
Example Instances
Only available in Dutch
| MedicatieGebruik DatumTijd | GebruikIndicator | VolgensAfspraak Indicator | Medicatiegebruik Stoptype | Gebruiksperiode | Gebruiksproduct | ||
| Ingangsdatum | Einddatum | Gebruiksduur | ProductCode | ||||
| 3-6-2014 16:19:07 | Ja | Mei 2014 | 1 maand | Paracetamol tablet 500 mg | |||
| 11-9-2012 17:21:00 | Ja | Ja | 01-09-12 | 05-09-12 | Pantoprazol injpdr 40 mg fl | ||
| 19-9-2014 4:12:11 | Nee | Nee | Definitief | 17-09-14 | Dalteparine 2500 injvlst 12.500 ie/ml wwsp 0,2ml | ||
| RedenGebruik | RedenWijzigen OfStoppen Gebruik | GebruiksInstructie | |||||
| Omschrijving | ToedieningsWeg | Aanvullende instructie | Doseerinstructie | ||||
| Doseerduur | Dosering| Keerdosis | Toedieningsschema |Frequentie |Interval |Toedientijd |Weekdag |Dagdeel | |||||
| Pijn | In de maand mei heb ik regelmatig paracetamol gebruikt. | ||||||
| Ulcusprofylaxe | Vanaf 1 september 2012 gedurende 5 dagen 1x per dag om 8uur 40 mg (=1 st) | iv | 40 mg (=1 st) | 1x per dag om 8.00 uur | |||
| (Mogelijke) bijwerking | Tijdelijk gestopt vanwege toenemende bijwerkingen: duizeligheid en misselijkheid. | subcutaan | 2500 IE | 1x per dag om 18.00 uur | |||
Valuesets
MedicationUseStopTypeCodeList
| Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.9.11.1 |
| Conceptname | Conceptcode | Codesystem name | Codesystem OID | Description |
| Tijdelijk | 1 | Medicatieafspraak StopType | 2.16.840.1.113883.2.4.3.11.60.20.77.5.2.1 | Tijdelijke onderbreking van medicamenteuze behandeling (bijvoorbeeld tijdelijk stoppen gebruik vanwege operatie). |
| Definitief | 2 | Medicatie afspraak StopType | 2.16.840.1.113883.2.4.3.11.60.20.77.5.2.1 | Het staken van een bestaande medicamenteuze behandeling. |
ReasonForChangeOrDiscontinuationOfUseCodList
| Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.9.11.2 |
| Conceptname | Conceptcode | Codesystem name | Codesystem OID | Description |
| Medication commenced (finding) | 266709005 | SNOMED CT | 2.16.840.1.113883.6.96 | Starten medicamenteuze behandeling |
| Administration of drug or medicament contraindicated (situation) | 438833006 | SNOMED CT | 2.16.840.1.113883.6.96 | Contra-indicatie |
| Drug interaction (disorder) | 79899007 | SNOMED CT | 2.16.840.1.113883.6.96 | Interactie |
| Hypersensitivity condition (disorder) | 473010000 | SNOMED CT | 2.16.840.1.113883.6.96 | Overgevoeligheid |
| Geen of onvoldoende effect | 5 | Medicatieafspraak Reden | 2.16.840.1.113883.2.4.3.11.60.20.77.5.2.2 | Geen of onvoldoende effect |
| Te sterk effect | 6 | Medicatieafspraak Reden | 2.16.840.1.113883.2.4.3.11.60.20.77.5.2.2 | Te sterk effect |
| (Mogelijke) bijwerking | 7 | Medicatieafspraak Reden | 2.16.840.1.113883.2.4.3.11.60.20.77.5.2.2 | (Mogelijke) bijwerking |
| Toedieningsweg voldoet niet | 8 | Medicatieafspraak Reden | 2.16.840.1.113883.2.4.3.11.60.20.77.5.2.2 | Toedieningsweg voldoet niet |
| Indicatie vervallen | 9 | Medicatieafspraak Reden | 2.16.840.1.113883.2.4.3.11.60.20.77.5.2.2 | Indicatie vervallen |
| Beleidswijziging | 10 | Medicatieafspraak Reden | 2.16.840.1.113883.2.4.3.11.60.20.77.5.2.2 | Beleidswijziging |
| Admission to establishment (procedure) | 305335007 | SNOMED CT | 2.16.840.1.113883.6.96 | Opname |
| Wens patiënt | 12 | Medicatieafspraak Reden | 2.16.840.1.113883.2.4.3.11.60.20.77.5.2.2 | Wens patiënt |
| Volgens afspraak | 13 | Medicatieafspraak Reden | 2.16.840.1.113883.2.4.3.11.60.20.77.5.2.2 | Volgens afspraak |
| Hervatten beleid vorige voorschrijver | 14 | Medicatieafspraak Reden | 2.16.840.1.113883.2.4.3.11.60.20.77.5.2.2 | Hervatten beleid vorige voorschrijver |
| Geplande procedure | 15 | Medicatieafspraak Reden | 2.16.840.1.113883.2.4.3.11.60.20.77.5.2.2 | Procedure waaronder ingreep, interferentie met gepland labonderzoek, etc. |
| Overig | OTH | NullFlavour | 2.16.840.1.113883.5.1008 | Overig |
This information model in other releases
---
More on this information model
To exchange information based on health and care information models, additional, more technical specifications are required.
Not every environment can handle the same technical specifications. For this reason, there are several types of technical specifications:
- HL7® version 3 CDA compatible specifications, available through the Nictiz ART-DECOR® environment

- HL7® FHIR® compatible specifications, available through the Nictiz environment on the Simplifier FHIR

This information model is also available as pdf file ![]() or as spreadsheet
or as spreadsheet ![]()
About this information
The information in this wikipage is based on Prerelease 2017 #1
Conditions for use are located on the mainpage ![]()
This page is generated on 30/11/2017 13:37:32